Review Process
Each research project application will undergo two levels of pre-screening review, one for technical feasibility as a high-throughput screening project and the other for scientific merit. If a research project has already been reviewed and funded by CPRIT or the NIH, it will be considered scientifically qualified and will only be reviewed for technical feasibility to ensure compatibility with TxSACT resources. The Technical Review Committee will be comprised of the screening experts from each of the TxSACT screening programs.
If an application is technically qualified, a further review of Scientific merit will be conducted that includes criteria such as scientific evidence for the proposal, degree of innovation, impact on knowledge about the basic biology of cancer, likelihood in producing new molecules or drug combinations that could advance to development for clinical evaluation, potential commercialization and others. The scientific review will be provided by a sub-committee of experienced cancer researchers who serve as members of the Executive Committee for the overall program. Based on the recommendation of these two committees, the Director of the overall Program will either accept the proposal or return it to the investigator. Extensive feedback will be provided to investigators whose proposals are returned to support the development of a competitive reapplication.
Once a proposal is accepted, the Director and the project’s investigator will agree to a formal Screening Services Agreement that will specify their mutual expectations. These will include the research protocol, types of screens, the number and types of test articles to be screened, expected timetable, data analysis, storage and sharing issues and expectations for cost recovery.
Assay Types and Required Supporting Data
There are two types of projects that will be accepted by the Program.:
1. Projects that will utilize one of the standard cytoxicity assays that each of the screening centers offer for fundamental studies on the inhibition of cell proliferation and the induction of cell death.
2. Projects will involve customized assays developed by investigators to address specific aspects of cancer cell biology or function.
The standard cytotoxicity assays offered by the TxSACT New Target Development and Combinatorial screening programs will accommodate both single agent and synthetic lethality screens. The lethality screens can be performed using either siRNA or drugs and small molecules alone or in combination. Investigators wanting to use a standard assay must have one or more cell lines that exhibit a clear distinction between cells responding to treatment and those that do not. These assays are typically used to address questions of new target identification, resistance, chemoprevention and improvement to existing treatment paradigms. The information and data supplied by the investigator in the TxSACT application will address the extent of the characterization of the cell lines to be used and the appropriateness of the cell lines as a model system for addressing the goals of the project.
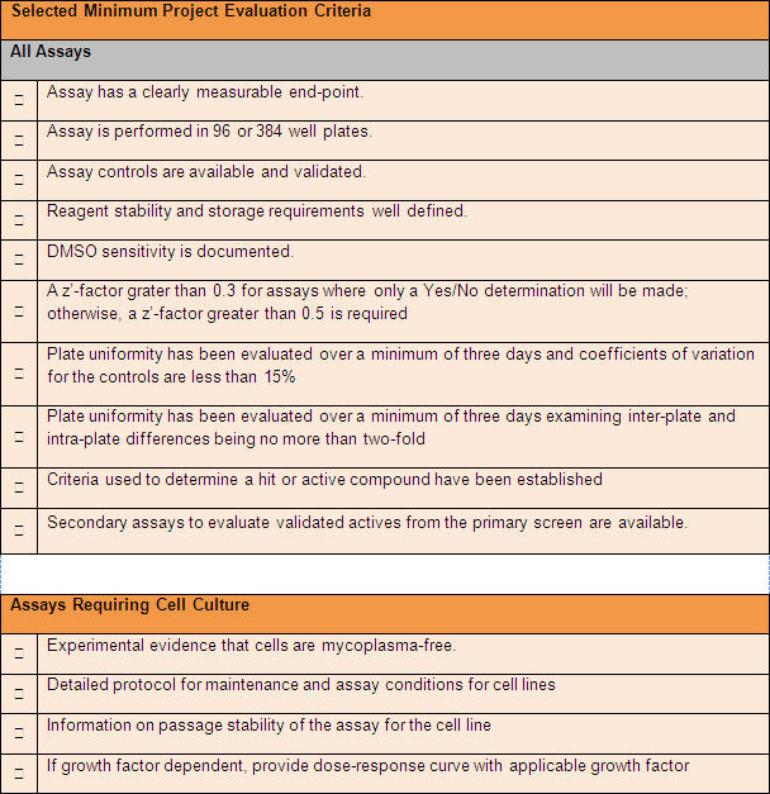
For those investigators with customized assays, the information requested and data requirements in the application are more extensive. Because the TxSACT program does not provide investigators with assay development services (these services can be obtained independently through the Gulf Coast Consortium for Chemical Genomics Program (GCC-CG) or the Therapeutics Institute for Drug and Diagnostic Development (TI3D), it is desirable for investigators to have demonstrated the feasibility of their proposed primary screening assay(s). This requested for customized assays will address the items listed in the table in the "The Application Review Process" section above.